The Company and Market Opportunity:
The regulatory approval and marketing strategy is to use SanFlow as a substitute for genetic therapy and transfusion therapy in children with sickle cell disease (SCD) to prevent stroke and severe painful vaso-occlusive crisis and to avoid the need for life- long dependence on chronic blood transfusion. Approval of SanFlow for SCD treatment as an orphan drug will open the major markets of SanFlow to the treatment of stroke, traumatic brain injury, hemorrhagic shock as well as an alternative to blood transfusion.
The Company is seeking strategic corporate partnerships to meet the un-met medical needs of these major markets. Regulatory approval with matching private equity investment with government funding are actively pursued at Orphan Drug Conference in 2019 and major academic Symposium, such as 2018 ASH meeting in San Diego.
The Drug:
FDA recently agreed that continued development of SanFlow, for critical care and transfusion medicine was justified based on multiple pre-clinical safety and efficacy results.
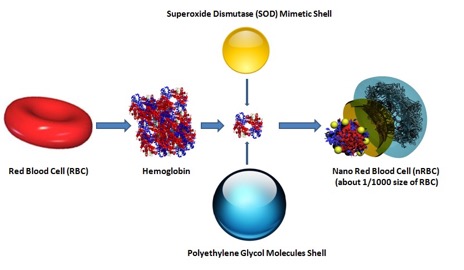
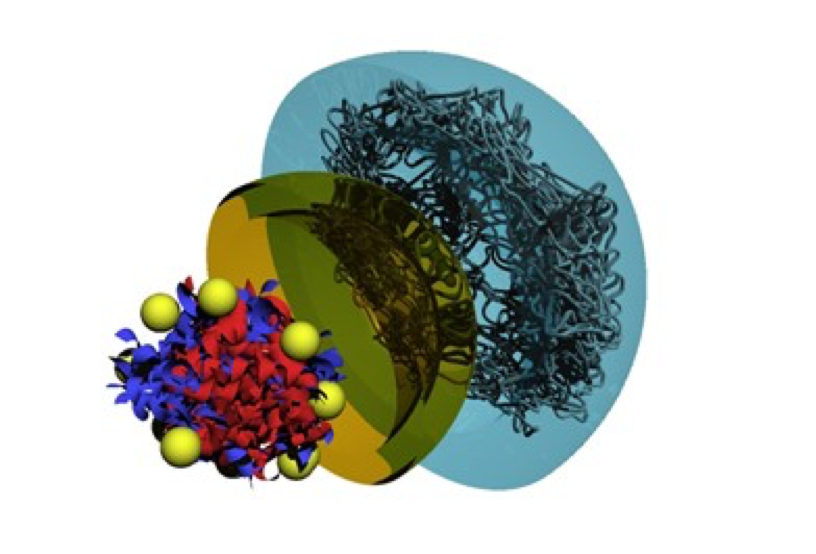
SanFlow in the form of a ~7 nm nanoparticle. The 3D structure in Diagram shown below as a dissected view with a core hemoglobin and its outer blue hydrated shell of covalently attached polyethylene glycol molecules and an inner superoxide dismutase (SOD) mimetic shell (gold) formed by covalently attached nitroxides with high spinning mobility (gold balls). The SOD mimetic shell prevents release of hemoglobin generated superoxide into the vascular space where it can cause vasoconstriction.
SanFlow flowing through a blood vessel are in the plasma phase as a nano medicine, can extend its SOD-mimetic activity to dismutate vascular over production of plasma superoxide resulting in the restoration of endogenous nitric oxide to restore blood flow to deliver life-saving oxygen for the prevention of ischemia, reperfusion and inflammation in sickle cell disease, stroke, traumatic brain injury, hemorrhagic shock as well as blood transfusion.

